M-A-U/iStock via Getty Images
Top-Line Summary
Fate Therapeutics (NASDAQ:FATE) is a cell therapy company attempting to harness CAR-T and NK cells to fight autoimmune diseases and cancer. After a skid lasting almost 2 years from highs of $110 a share, it remains difficult to see their path forward, considering their product candidates are still in early clinical trials or remain preclinical. There’s still a long road to hoe for FATE, and their large cash position is being eaten up very quickly, with glimmers of hope from their partnership with Ono. This translates into a stock that is very speculative at best, and one I would not feel comfortable getting into at this time.
Pipeline Overview
FATE is mainly focused on oncology at this time, with 2 clinical candidate products it is shepherding through phase 1 trials: FT576 and FT819.
FT576
FT576 is an expanded natural killer (NK) cell immunotherapy that is designed with a chimeric antigen receptor targeting BCMA, which has been one of the hot targets for multiple myeloma over the last several years. Several other modifications are designed to enhance the activation of the NK cells once in the body, as well as reduce cell death by other NK cells that might still be present.
We saw an interim readout of the phase 1 trial at last year’s ASH meeting in poster form. In particular, combining FT576 with daratumumab led to impressive tumor control in mice engrafted with multiple myeloma cells:
FT576 efficacy in MM-engrafted mice (ASH 2022 Poster Presentation)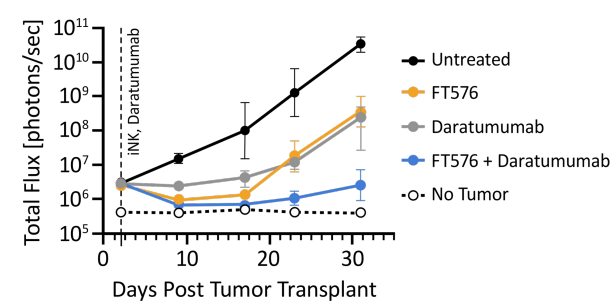
In patients with relapsed/refractory MM, FT576 was well tolerated, with no classic toxicities of CAR T-cell therapy like CRS or ICANS being observed. No patient developed graft-versus-host disease, either. High-grade diarrhea and low cell counts were observed, though. One out of 8 patients achieved a very good partial response, although this was in the small group of patients who received additional anti-CD38 therapy, which is hoped to improved cell survival. This is a good, if early, sign that things are progressing as intended for this program. However, it’s definitely early days to get excited. FATE is continuing to conduct this phase 1 trial, and I’m sure we’ll hear more in the coming months and years.
At this time, no later-stage trial has been announced for FT576, although their eyes are surely fixed on that target.
FT819
The other program that FATE is advancing is a novel CD19 CAR T-cell therapy, FT819. This is intended to be differentiated from approved CD19 CAR T-cell products by being off-the-shelf, as well as having a knockout of T cell receptor, which is understood to reduce the risk of graft-versus-host disease further.
Side note: The risk of GVHD is a serious concern for an “off the shelf” product like this one, since it would be akin to an allogeneic stem cell transplant. Currently, approved CD19 CAR T-cell therapies all come from the patient’s own T cells, making these therapies basically autologous transplants, which do not carry much risk of GVHD.
FATE provided a look into the ongoing phase 1 trial investigating FT819 at ASH 2022. This study is testing 2 different doses of cells to try and arrive at a recommended phase 2 dose, as well as learn more about the safety and efficacy of this therapy.
The therapy was well tolerated in the 15 patients with DLCBL or FL included in this study. Cytokine release syndrome was limited to grade 2 in severity, and the company observed no grade 3 or higher adverse events attributed to FT819 (although one patient died of sepsis, which was not deemed related to study therapy).
4 out of the 15 patients achieved a response to therapy, including 3 complete responses. This is a remarkable signal given that these patients were heavily pretreated or had high-risk features like Richter’s transformation.
Dose escalation is ongoing for this phase 1 study. FATE has not announced plans for other trials at the time of this writing.
FT825
In general, I don’t look much to preclinical products when providing overviews of these companies. Mainly this is to keep the size of these articles from ballooning out of control with what you might call “science projects.” They could be de-emphasized or eliminated at any time.
However, FT825 carries a special place for FATE, since it’s currently the only partnered program the company is continuing to develop. In a deal inked in 2018, Ono Pharmaceuticals (OTCPK:OPHLF) joined FATE to develop CAR T-cell products targeted at solid tumors. FT825 is the flagship of this partnership, with the main target of interest being HER2.
This is, of course, a massively important target in solid tumor oncology, touching breast cancer, stomach cancer, lung cancer, and colorectal cancer, among others.
Financial Outlook
As of the Q2 filing, FATE held $46.8 million in cash and equivalents, along with $331 million in short-term investments. Total current assets were $392 million, making this company positively flush with cash.
However, this faces off against rather massive costs, even after their big reset following the JNJ deal termination. Total operating expenses for Q2 2023 were $63.5 million, which is markedly down from $101.7 million in Q2 2022, but it’s still a big outlay.
At this burn rate, assuming they can keep their costs fixed, the company would have somewhere in the realm of 6 quarters of cash and short-term equivalents on hand to finance their activities, taking them optimistically into 2025. In their most recent earnings call, the company stated that they feel this cash runway will take them into the second half of 2025. Depending on how they manage their expenses, including stock-based compensation (which accounted for $12.9 million of their reported net loss), this might be a reasonable estimate.
Strengths and Risks
Anyone who has followed FATE knows that the major downward catalyst for the company in 2023 has been the termination of their partnership with Janssen. This capped off a steep decline that began in 2021 after a meteoric rise in share price.
This is a first, fast red flag: FATE has been able to drive up some serious hype behind interesting early-stage science. This is due in part to getting caught up in ARK frenzy. That’s over now as of January, since the ETF doesn’t hold FATE any more. The second red flag: no more partnership with Janssen and the deep pockets that could be used to fund massively expensive research and development. That’s definitively done, too.
They do have the ongoing collaboration with Ono, which was amended in June 2022 to expand the scope of their arrangement. FATE is eligible for over $800 million in milestone payments toward the development of 2 candidate products, and Ono exercised the option for one of these candidates, FT825, back in November of 2022.
This is a valuable step forward, but the ongoing revenue FATE is recognizing amounts to offsetting costs in the region of $1 to $3 million per quarter. It’s a valuable offset, but still does not come close to meeting the operating expenses that exceed $60 million per quarter.
That cash burn rate is the biggest risk factor in my mind. It just doesn’t align with where the company is in its clinical developments. To date, no CAR T therapy has been approved based on phase 1 results, and the first CAR T cell therapy to be approved took something like 3 years from its first publication (Maude et al 2014 is a seminal paper in cell therapy) before getting accelerated approval based on phase 2 results.
And those phase 1 results were spectacular. What we’ve seen so far from FATE has been more like what you’d expect from phase 1 studies: kind of exciting, no big surprises. The tolerability does look quite good, but these are still small patient groups, and you don’t typically see rapid approvals based only on improved safety.
Long story short? I don’t see anything from FATE so far that says this company has a drug on the market before 2026 at the very earliest, based on how CAR T cell therapies have typically been developed. And they have extra hurdles to clear here since the NK cell-based approach is so novel. The FDA will want a lot of due diligence to ensure safety.
Bottom Line Summary
FATE has in store some very interesting scientific premise, some promising early-stage clinical data, a deep cash reserve, and a massive expenditure to move their products forward.
Right now, barring some major, unexpected catalyst, I don’t think FATE is going to be making any big moves up in 2023. Likely they will remain steady or continue a slow decline through 2024, as well, with the only news I can foresee being updates to the phase 1 trials and initiation/planning of phase 2 studies. But these are not really catalysts that drive value, since they’re exactly what the company needs to do.
At this time, the only reason to buy into FATE is to try to be in on the ground floor before a major positive surprise event, and that’s for investors with a higher risk tolerance than I have. I’m rating this a sell until they find a way to stem the bleeding and continue to show progress in their trials. But they’re also a company worth putting on your watch list.
Editor’s Note: This article discusses one or more securities that do not trade on a major U.S. exchange. Please be aware of the risks associated with these stocks.
Credit: Source link